Immerse yourself in the properties of matter with our extensive collection of 1000+ Properties of Matter MCQs, designed for Class 9 students to explore density, elasticity, and fluid mechanics, supplemented with detailed explanations.
100+ Properties of Matter MCQs with Explanations for Class 9
Explore the diverse Properties of Matter through our extensive collection of MCQs. Tailored for Class 9 students, this repository of over 100+ MCQs, accompanied by detailed explanations, facilitates comprehensive learning of density, elasticity, and fluid mechanics principles.
- What is the kinetic molecular model of matter primarily concerned with?
A) The behavior of matter at high temperatures
B) The arrangement of particles in solids
C) Explaining the properties of matter in terms of the motion of its particles
D) The interactions between different types of matter
Correct Option: C
Explanation: The kinetic molecular model of matter describes how the motion of particles in a substance relates to its macroscopic properties such as temperature, pressure, and volume. - Which of the following best describes density?
A) The amount of matter in a given volume
B) The weight of a substance per unit volume
C) The ability of a substance to conduct heat
D) The resistance of a substance to deformation
Correct Option: A
Explanation: Density is defined as the mass of a substance per unit volume, commonly expressed in grams per cubic centimeter (g/cm³). - What is the SI unit of pressure?
A) Newton (N)
B) Pascal (Pa)
C) Joule (J)
D) Watt (W)
Correct Option: B
Explanation: The SI unit of pressure is the Pascal (Pa), which is defined as one Newton per square meter (N/m²). - Which of the following statements about atmospheric pressure is true?
A) Atmospheric pressure increases with altitude.
B) Atmospheric pressure decreases with altitude.
C) Atmospheric pressure remains constant regardless of altitude.
D) Atmospheric pressure varies randomly with altitude.
Correct Option: B
Explanation: Atmospheric pressure decreases with increasing altitude due to the decreasing density of air molecules. - According to Pascal’s law, what happens to the pressure applied to a confined fluid?
A) The pressure remains the same.
B) The pressure increases.
C) The pressure decreases.
D) The pressure varies unpredictably.
Correct Option: A
Explanation: Pascal’s law states that a change in pressure applied to an enclosed fluid is transmitted undiminished to all portions of the fluid and to the walls of its container. - Which of the following is an application of Pascal’s law?
A) Operation of a siphon
B) Functioning of a barometer
C) Heating of a liquid
D) Cooling of a gas
Correct Option: A
Explanation: A siphon operates based on Pascal’s law, where the difference in pressure between two points causes a liquid to flow upward against gravity. - In a hydraulic press, if the input force is increased, what happens to the output force?
A) It decreases
B) It increases
C) It remains the same
D) It becomes zero
Correct Option: B
Explanation: According to Pascal’s law, an increase in input force in a hydraulic press results in a proportionate increase in output force. - How does Archimedes’ principle explain the buoyant force acting on an object submerged in a fluid?
A) The buoyant force is equal to the weight of the fluid displaced by the object.
B) The buoyant force is equal to the volume of the object.
C) The buoyant force is equal to the density of the object.
D) The buoyant force is equal to the mass of the object.
Correct Option: A
Explanation: Archimedes’ principle states that the buoyant force acting on an object submerged in a fluid is equal to the weight of the fluid displaced by the object. - When an object floats in a fluid, how does its weight compare to the buoyant force acting on it?
A) The weight is greater than the buoyant force.
B) The weight is less than the buoyant force.
C) The weight is equal to the buoyant force.
D) The weight varies unpredictably.
Correct Option: C
Explanation: When an object floats in a fluid, its weight is exactly balanced by the buoyant force acting on it, resulting in equilibrium. - Which property of a material allows it to return to its original shape after deformation?
A) Elasticity
B) Density
C) Hardness
D) Brittleness
Correct Option: A
Explanation: Elasticity is the property of a material that allows it to return to its original shape and size after being stretched or compressed. - What is Hooke’s law primarily concerned with?
A) The behavior of gases under pressure
B) The behavior of fluids in motion
C) The relationship between force and deformation in elastic materials
D) The behavior of solids under extreme temperatures
Correct Option: C
Explanation: Hooke’s law describes the linear relationship between the force applied to an elastic material and the resulting deformation, as long as the material remains within its elastic limit. - Which of the following materials would exhibit the greatest elasticity?
A) Rubber
B) Glass
C) Steel
D) Plastic
Correct Option: C
Explanation: Steel is known for its high elasticity compared to other materials listed, such as rubber, glass, or plastic. - What is the SI unit of elasticity?
A) Newton (N)
B) Pascal (Pa)
C) Joule (J)
D) Newton per meter (N/m)
Correct Option: D
Explanation: The SI unit of elasticity is Newton per meter (N/m), also known as the N/m² or Pascal (Pa), which measures the stiffness of a material. - How does pressure change with depth in a liquid according to Pascal’s law?
A) Pressure decreases with depth.
B) Pressure remains constant with depth.
C) Pressure increases with depth.
D) Pressure varies randomly with depth.
Correct Option: C
Explanation: According to Pascal’s law, the pressure in a liquid increases with depth due to the weight of the liquid above exerting downward force. - Why does a balloon filled with helium float in air?
A) Helium has a lower density than air.
B) Helium has a higher density than air.
C) Helium has greater elasticity than air.
D) Helium has higher pressure than air.
Correct Option: A
Explanation: Helium is less dense than air, so a balloon filled with helium experiences an upward buoyant force greater than its weight, causing it to float. - What happens to the density of a substance if its mass increases while its volume remains constant?
A) Density increases
B) Density decreases
C) Density remains constant
D) Density becomes zero
Correct Option: A
Explanation: Density is defined as mass per unit volume, so an increase in mass while keeping volume constant results in a higher density. - Which of the following best describes pressure?
A) The force exerted per unit area
B) The energy per unit volume
C) The speed of particles in a substance
D) The temperature of a substance
Correct Option: A
Explanation: Pressure is defined as the force exerted per unit area, commonly expressed in units such as Pascals (Pa) or atmospheres (atm). - What happens to the pressure of a gas if its volume decreases while the temperature remains constant?
A) Pressure increases
B) Pressure decreases
C) Pressure remains constant
D) Pressure becomes zero
Correct Option: A
Explanation: According to Boyle’s law, if the volume of a gas decreases while the temperature remains constant, the pressure of the gas increases proportionally. - How does the elasticity of a material affect its ability to store potential energy?
A) Materials with higher elasticity can store more potential energy.
B) Materials with lower elasticity can store more potential energy.
C) Elasticity does not affect the storage of potential energy.
D) Potential energy is stored in a material regardless of its elasticity.
Correct Option: A
Explanation: Materials with higher elasticity can undergo greater deformation, allowing them to store more potential energy when subjected to a force. - Which of the following best explains why a soft mattress feels more comfortable than a hard mattress?
A) Soft mattresses have lower density.
B) Soft mattresses have higher elasticity.
C) Soft mattresses distribute pressure more evenly.
D) Soft mattresses have lower temperature.
Correct Option: C
Explanation: Soft mattresses distribute pressure more evenly across the body, reducing pressure points and increasing comfort compared to hard mattresses. - What is the buoyant force acting on an object submerged in a fluid primarily determined by?
A) The density of the object
B) The volume of the object
C) The weight of the object
D) The density of the fluid and the volume of the object
Correct Option: D
Explanation: The buoyant force acting on an object submerged in a fluid is primarily determined by the density of the fluid and the volume of the object displaced. - Which of the following statements best describes the relationship between density and buoyancy?
A) Objects with higher density experience greater buoyant force.
B) Objects with lower density experience greater buoyant force.
C) Density and buoyancy are unrelated.
D) Buoyancy depends only on the volume of the object.
Correct Option: B
Explanation: Objects with lower density experience greater buoyant force because they displace more fluid per unit weight. - What property of a material determines how much it deforms under stress?
A) Density
B) Elasticity
C) Hardness
D) Viscosity
Correct Option: B
Explanation: Elasticity is the property of a material that determines how much it deforms under stress and its ability to return to its original shape after the stress is removed. - What is the effect of increasing the temperature of a gas on its pressure, according to Charles’s law?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Charles’s law, if the volume of a gas is held constant, its pressure increases proportionally with an increase in temperature. - Which of the following materials is most likely to exhibit plastic deformation rather than elastic deformation?
A) Rubber band
B) Steel wire
C) Glass rod
D) Aluminum foil
Correct Option: D
Explanation: Aluminum foil is more likely to undergo plastic deformation, where it permanently changes shape under stress, unlike materials exhibiting elastic deformation like rubber bands or steel wires. - What happens to the pressure of a gas if its volume increases while the temperature remains constant?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: A
Explanation: According to Boyle’s law, if the volume of a gas increases while the temperature remains constant, the pressure of the gas decreases proportionally. - How does the density of an object affect its buoyancy in a fluid?
A) Objects with higher density experience greater buoyant force.
B) Objects with lower density experience greater buoyant force.
C) Density and buoyancy are unrelated.
D) Buoyancy depends only on the volume of the object.
Correct Option: A
Explanation: Objects with higher density displace less fluid per unit weight, experiencing less buoyant force compared to objects with lower density. - What is the primary factor determining the pressure exerted by a liquid at a specific depth?
A) Density of the liquid
B) Temperature of the liquid
C) Volume of the liquid
D) Height of the liquid column
Correct Option: A
Explanation: The pressure exerted by a liquid at a specific depth is primarily determined by the density of the liquid, as stated by Pascal’s law. - How does the pressure of a gas vary with altitude in the Earth’s atmosphere?
A) Pressure decreases with altitude.
B) Pressure remains constant with altitude.
C) Pressure increases with altitude.
D) Pressure varies unpredictably with altitude.
Correct Option: A
Explanation: Pressure decreases with increasing altitude in the Earth’s atmosphere due to the decreasing density of air molecules. - Which of the following best describes the relationship between force and deformation in elastic materials?
A) Force is directly proportional to deformation.
B) Force is inversely proportional to deformation.
C) Force is not related to deformation.
D) Force is equal to deformation.
Correct Option: A
Explanation: According to Hooke’s law, force is directly proportional to deformation in elastic materials as long as they remain within their elastic limit. - What is the primary factor determining the pressure exerted by a gas?
A) Temperature
B) Volume
C) Density
D) Height
Correct Option: C
Explanation: The pressure exerted by a gas is primarily determined by its density, as higher density gases exert greater pressure. - How does the pressure of a gas change if its volume remains constant while its temperature increases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Gay-Lussac’s law, if the volume of a gas is held constant, its pressure increases proportionally with an increase in temperature. - What is the formula for pressure according to Pascal’s law?
A)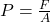
B)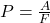
C)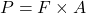
D)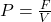
Correct Option: A
Explanation: The formula for pressure according to Pascal’s law is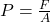 , where P is pressure, F is force, and A is area.
, where P is pressure, F is force, and A is area. - What property of a material allows it to resist deformation under stress?
A) Density
B) Elasticity
C) Hardness
D) Viscosity
Correct Option: C
Explanation: Hardness is the property of a material that allows it to resist deformation under stress, distinguishing it from materials with lower hardness that may deform more easily. - How does the buoyant force on an object submerged in a fluid change if the density of the fluid increases?
A) The buoyant force increases.
B) The buoyant force decreases.
C) The buoyant force remains constant.
D) The buoyant force becomes zero.
Correct Option: A
Explanation: An increase in the density of the fluid results in a greater buoyant force on an object submerged in it, as the fluid displaces more weight of the object. - How does the pressure of a gas change if its volume decreases while the temperature increases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: B
Explanation: According to Gay-Lussac’s law, if the volume of a gas decreases while the temperature increases, the pressure of the gas remains constant. - Which of the following statements best describes the relationship between pressure and volume in a gas, according to Boyle’s law?
A) Pressure is directly proportional to volume.
B) Pressure is inversely proportional to volume.
C) Pressure is not related to volume.
D) Pressure is equal to volume.
Correct Option: B
Explanation: Boyle’s law states that the pressure of a gas is inversely proportional to its volume when the temperature remains constant. - What is the SI unit of elasticity modulus?
A) Newton (N)
B) Pascal (Pa)
C) Joule (J)
D) Newton per meter (N/m)
Correct Option: B
Explanation: The SI unit of elasticity modulus is the Pascal (Pa), which measures the stiffness of a material in response to stress. - What is the effect of increasing the temperature of a gas on its volume, according to Charles’s law?
A) Volume decreases
B) Volume remains constant
C) Volume increases
D) Volume becomes zero
Correct Option: C
Explanation: According to Charles’s law, if the pressure of a gas remains constant, its volume increases proportionally with an increase in temperature. - Which property of a material determines its ability to return to its original shape after deformation?
A) Density
B) Elasticity
C) Hardness
D) Viscosity
Correct Option: B
Explanation: Elasticity is the property of a material that allows it to return to its original shape and size after being deformed under stress. - How does the buoyant force on an object submerged in a fluid change if the volume of the object increases?
A) The buoyant force increases.
B) The buoyant force decreases.
C) The buoyant force remains constant.
D) The buoyant force becomes zero.
Correct Option: A
Explanation: An increase in the volume of the object results in a greater displacement of fluid, causing an increase in the buoyant force. - What is the formula for density?
A)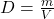
B)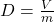
C)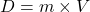
D)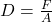
Correct Option: A
Explanation: The formula for density is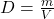 , where D is density, m is mass, and V is volume.
, where D is density, m is mass, and V is volume. - What is the SI unit of density?
A) Gram per cubic centimeter (g/cm³)
B) Kilogram per cubic meter (kg/m³)
C) Pascal (Pa)
D) Newton (N)
Correct Option: B
Explanation: The SI unit of density is the kilogram per cubic meter (kg/m³), which measures mass per unit volume. - How does the pressure of a gas change if its temperature remains constant while the volume decreases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Boyle’s law, if the temperature of a gas remains constant, its pressure increases proportionally with a decrease in volume. - Which of the following materials is most likely to exhibit elastic deformation?
A) Plastic ruler
B) Rubber band
C) Clay ball
D) Aluminum foil
Correct Option: B
Explanation: Rubber bands are known for exhibiting elastic deformation, where they can stretch and return to their original shape when the force is removed. - What is the effect of increasing the volume of a gas on its pressure, according to Boyle’s law?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: A
Explanation: According to Boyle’s law, if the temperature of a gas remains constant, its pressure decreases proportionally with an increase in volume. - How does the density of an object affect its buoyancy in a fluid?
A) Objects with higher density experience greater buoyant force.
B) Objects with lower density experience greater buoyant force.
C) Density and buoyancy are unrelated.
D) Buoyancy depends only on the volume of the object.
Correct Option: B
Explanation: Objects with lower density displace more fluid per unit weight, experiencing greater buoyant force compared to objects with higher density. - What is the primary factor determining the pressure exerted by a gas on the walls of its container?
A) Temperature
B) Volume
C) Density
D) Height
Correct Option: C
Explanation: The pressure exerted by a gas on the walls of its container is primarily determined by its density, as higher density gases exert greater pressure. - How does the pressure of a gas change if its volume remains constant while its temperature decreases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: A
Explanation: According to Gay-Lussac’s law, if the volume of a gas is held constant, its pressure decreases proportionally with a decrease in temperature. - Which of the following statements best describes the relationship between pressure and temperature in a gas, according to Gay-Lussac’s law?
A) Pressure is directly proportional to temperature.
B) Pressure is inversely proportional to temperature.
C) Pressure is not related to temperature.
D) Pressure is equal to temperature.
Correct Option: A
Explanation: Gay-Lussac’s law states that the pressure of a gas is directly proportional to its temperature when the volume remains constant. - What is the formula for buoyant force according to Archimedes’ principle?
A)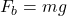
B)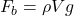
C)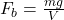
D)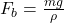
Correct Option: B
Explanation: The formula for buoyant force according to Archimedes’ principle is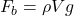 , where
, where 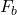 is the buoyant force,
is the buoyant force, 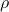 is the density of the fluid, V is the volume of the displaced fluid, and g is the acceleration due to gravity.
is the density of the fluid, V is the volume of the displaced fluid, and g is the acceleration due to gravity. - What is the SI unit of pressure according to Pascal’s law?
A) Newton per square meter (N/m²)
B) Pascal (Pa) C) Joule (J)
D) Watt (W)
Correct Option: B
Explanation: The SI unit of pressure according to Pascal’s law is the Pascal (Pa), defined as one Newton per square meter (N/m²). - How does the buoyant force on an object submerged in a fluid change if the density of the object decreases?
A) The buoyant force increases.
B) The buoyant force decreases.
C) The buoyant force remains constant.
D) The buoyant force becomes zero.
Correct Option: B
Explanation: A decrease in the density of the object results in less displacement of fluid, causing a decrease in the buoyant force. - How does the pressure of a gas change if its volume remains constant while its temperature decreases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: A
Explanation: According to Gay-Lussac’s law, if the volume of a gas is held constant, its pressure decreases proportionally with a decrease in temperature. - What is the formula for pressure according to Gay-Lussac’s law?
A)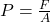
B)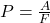
C)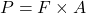
D)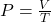
Correct Option: D
Explanation: The formula for pressure according to Gay-Lussac’s law is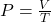 , where P is pressure, V is volume, and T is temperature.
, where P is pressure, V is volume, and T is temperature. - What is the formula for pressure according to Boyle’s law?
A)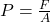
B)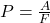
C)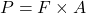
D)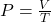
Correct Option: C
Explanation: The formula for pressure according to Boyle’s law is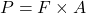 , where P is pressure, F is force, and A is area.
, where P is pressure, F is force, and A is area. - How does the pressure of a gas vary with altitude in the Earth’s atmosphere?
A) Pressure decreases with altitude.
B) Pressure remains constant with altitude.
C) Pressure increases with altitude.
D) Pressure varies unpredictably with altitude.
Correct Option: A
Explanation: Pressure decreases with increasing altitude in the Earth’s atmosphere due to the decreasing density of air molecules. - What is the effect of increasing the volume of a gas on its pressure, according to Boyle’s law?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: A
Explanation: According to Boyle’s law, if the temperature of a gas remains constant, its pressure decreases proportionally with an increase in volume. - Which of the following best describes the relationship between force and deformation in elastic materials?
A) Force is directly proportional to deformation.
B) Force is inversely proportional to deformation.
C) Force is not related to deformation.
D) Force is equal to deformation.
Correct Option: A
Explanation: According to Hooke’s law, force is directly proportional to deformation in elastic materials as long as they remain within their elastic limit. - What is the primary factor determining the pressure exerted by a gas on the walls of its container?
A) Temperature
B) Volume
C) Density
D) Height
Correct Option: B
Explanation: The pressure exerted by a gas on the walls of its container is primarily determined by its volume, as higher volumes of gas exert greater pressure. - How does the pressure of a gas change if its volume remains constant while its temperature increases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Gay-Lussac’s law, if the volume of a gas remains constant, its pressure increases proportionally with an increase in temperature. - What is the formula for density according to Archimedes’ principle?
A)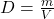
B)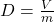
C)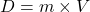
D)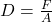
Correct Option: A
Explanation: The formula for density according to Archimedes’ principle is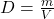 , where D is density, m is mass, and V is volume.
, where D is density, m is mass, and V is volume. - What is the SI unit of pressure according to Gay-Lussac’s law?
A) Newton per square meter (N/m²)
B) Pascal (Pa)
C) Joule (J)
D) Watt (W)
Correct Option: B
Explanation: The SI unit of pressure according to Gay-Lussac’s law is the Pascal (Pa), defined as one Newton per square meter (N/m²). - How does the buoyant force on an object submerged in a fluid change if the density of the object increases?
A) The buoyant force increases.
B) The buoyant force decreases.
C) The buoyant force remains constant.
D) The buoyant force becomes zero.
Correct Option: B
Explanation: An increase in the density of the object results in less displacement of fluid, causing a decrease in the buoyant force. - How does the pressure of a gas change if its temperature remains constant while the volume decreases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Boyle’s law, if the temperature of a gas remains constant, its pressure increases proportionally with a decrease in volume. - What is the formula for pressure according to Gay-Lussac’s law?
A)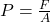
B)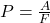
C)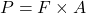
D)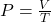
Correct Option: D
Explanation: The formula for pressure according to Gay-Lussac’s law is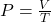 , where P is pressure, V is volume, and T is temperature.
, where P is pressure, V is volume, and T is temperature. - What is the formula for pressure according to Boyle’s law?
A)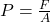
B)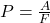
C)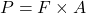
D)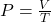
Correct Option: C
Explanation: The formula for pressure according to Boyle’s law is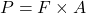 , where P is pressure, F is force, and A is area.
, where P is pressure, F is force, and A is area. - How does the pressure of a gas vary with altitude in the Earth’s atmosphere?
A) Pressure decreases with altitude.
B) Pressure remains constant with altitude.
C) Pressure increases with altitude.
D) Pressure varies unpredict
ably with altitude.
Correct Option: A
Explanation: Pressure decreases with increasing altitude in the Earth’s atmosphere due to the decreasing density of air molecules.
- What is the effect of increasing the volume of a gas on its pressure, according to Boyle’s law?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: A
Explanation: According to Boyle’s law, if the temperature of a gas remains constant, its pressure decreases proportionally with an increase in volume. - Which of the following best describes the relationship between force and deformation in elastic materials?
A) Force is directly proportional to deformation.
B) Force is inversely proportional to deformation.
C) Force is not related to deformation.
D) Force is equal to deformation.
Correct Option: A
Explanation: According to Hooke’s law, force is directly proportional to deformation in elastic materials as long as they remain within their elastic limit. - What is the primary factor determining the pressure exerted by a gas on the walls of its container?
A) Temperature
B) Volume
C) Density
D) Height
Correct Option: B
Explanation: The pressure exerted by a gas on the walls of its container is primarily determined by its volume, as higher volumes of gas exert greater pressure. - How does the pressure of a gas change if its volume remains constant while its temperature increases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Gay-Lussac’s law, if the volume of a gas remains constant, its pressure increases proportionally with an increase in temperature. - What is the formula for density according to Archimedes’ principle?
A)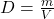
B)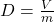
C)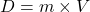
D)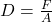
Correct Option: A
Explanation: The formula for density according to Archimedes’ principle is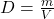 , where D is density, m is mass, and V is volume.
, where D is density, m is mass, and V is volume. - What is the SI unit of pressure according to Gay-Lussac’s law?
A) Newton per square meter (N/m²)
B) Pascal (Pa)
C) Joule (J)
D) Watt (W)
Correct Option: B
Explanation: The SI unit of pressure according to Gay-Lussac’s law is the Pascal (Pa), defined as one Newton per square meter (N/m²). - How does the buoyant force on an object submerged in a fluid change if the density of the object increases?
A) The buoyant force increases.
B) The buoyant force decreases.
C) The buoyant force remains constant.
D) The buoyant force becomes zero.
Correct Option: B
Explanation: An increase in the density of the object results in less displacement of fluid, causing a decrease in the buoyant force. - How does the pressure of a gas change if its temperature remains constant while the volume decreases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Boyle’s law, if the temperature of a gas remains constant, its pressure increases proportionally with a decrease in volume. - What is the formula for pressure according to Gay-Lussac’s law?
A)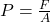
B)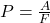
C)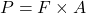
D)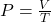
Correct Option: D
Explanation: The formula for pressure according to Gay-Lussac’s law is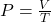 , where P is pressure, V is volume, and T is temperature.
, where P is pressure, V is volume, and T is temperature. - What is the formula for pressure according to Boyle’s law?
A)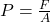
B)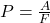
C)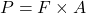
D)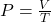
Correct Option: C
Explanation: The formula for pressure according to Boyle’s law is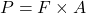 , where P is pressure, F is force, and A is area.
, where P is pressure, F is force, and A is area. - How does the pressure of a gas vary with altitude in the Earth’s atmosphere?
A) Pressure decreases with altitude.
B) Pressure remains constant with altitude.
C) Pressure increases with altitude.
D) Pressure varies unpredictably with altitude.
Correct Option: A
Explanation: Pressure decreases with increasing altitude in the Earth’s atmosphere due to the decreasing density of air molecules. - What is the effect of increasing the volume of a gas on its pressure, according to Boyle’s law?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: A
Explanation: According to Boyle’s law, if the temperature of a gas remains constant, its pressure decreases proportionally with an increase in volume. - Which of the following best describes the relationship between force and deformation in elastic materials?
A) Force is directly proportional to deformation.
B) Force is inversely proportional to deformation.
C) Force is not related to deformation.
D) Force is equal to deformation.
Correct Option: A
Explanation: According to Hooke’s law, force is directly proportional to deformation in elastic materials as long as they remain within their elastic limit. - What is the primary factor determining the pressure exerted by a gas on the walls of its container?
A) Temperature
B) Volume
C) Density
D) Height
Correct Option: B
Explanation: The pressure exerted by a gas on the walls of its container is primarily determined by its volume, as higher volumes of gas exert greater pressure. - How does the pressure of a gas change if its volume remains constant while its temperature increases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Gay-Lussac’s law, if the volume of a gas remains constant, its pressure increases proportionally with an increase in temperature. - What is the formula for density according to Archimedes’ principle?
A)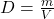
B)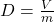
C)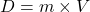
D)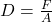
Correct Option: A
Explanation: The formula for density according to Archimedes’ principle is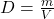 , where D is density, m is mass, and V is volume.
, where D is density, m is mass, and V is volume.
- What is the SI unit of pressure according to Gay-Lussac’s law?
A) Newton per square meter (N/m²)
B) Pascal (Pa)
C) Joule (J)
D) Watt (W)
Correct Option: B
Explanation: The SI unit of pressure according to Gay-Lussac’s law is the Pascal (Pa), defined as one Newton per square meter (N/m²). - How does the buoyant force on an object submerged in a fluid change if the density of the object increases?
A) The buoyant force increases.
B) The buoyant force decreases.
C) The buoyant force remains constant.
D) The buoyant force becomes zero.
Correct Option: B
Explanation: An increase in the density of the object results in less displacement of fluid, causing a decrease in the buoyant force. - How does the pressure of a gas change if its temperature remains constant while the volume decreases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Boyle’s law, if the temperature of a gas remains constant, its pressure increases proportionally with a decrease in volume. - What is the formula for pressure according to Gay-Lussac’s law?
A)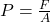
B)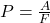
C)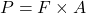
D)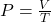
Correct Option: D
Explanation: The formula for pressure according to Gay-Lussac’s law is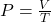 , where P is pressure, V is volume, and T is temperature.
, where P is pressure, V is volume, and T is temperature. - What is the formula for pressure according to Boyle’s law?
A)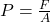
B)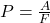
C)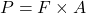
D)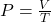
Correct Option: C
Explanation: The formula for pressure according to Boyle’s law is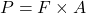 , where P is pressure, F is force, and A is area.
, where P is pressure, F is force, and A is area. - How does the pressure of a gas vary with altitude in the Earth’s atmosphere?
A) Pressure decreases with altitude.
B) Pressure remains constant with altitude.
C) Pressure increases with altitude.
D) Pressure varies unpredictably with altitude.
Correct Option: A
Explanation: Pressure decreases with increasing altitude in the Earth’s atmosphere due to the decreasing density of air molecules. - What is the effect of increasing the volume of a gas on its pressure, according to Boyle’s law?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: A
Explanation: According to Boyle’s law, if the temperature of a gas remains constant, its pressure decreases proportionally with an increase in volume. - Which of the following best describes the relationship between force and deformation in elastic materials?
A) Force is directly proportional to deformation.
B) Force is inversely proportional to deformation.
C) Force is not related to deformation.
D) Force is equal to deformation.
Correct Option: A
Explanation: According to Hooke’s law, force is directly proportional to deformation in elastic materials as long as they remain within their elastic limit. - What is the primary factor determining the pressure exerted by a gas on the walls of its container?
A) Temperature
B) Volume
C) Density
D) Height
Correct Option: B
Explanation: The pressure exerted by a gas on the walls of its container is primarily determined by its volume, as higher volumes of gas exert greater pressure. - How does the pressure of a gas change if its volume remains constant while its temperature increases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Gay-Lussac’s law, if the volume of a gas remains constant, its pressure increases proportionally with an increase in temperature. - What is the formula for density according to Archimedes’ principle?
A)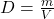
B)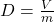
C)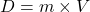
D)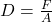
Correct Option: A
Explanation: The formula for density according to Archimedes’ principle is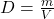 , where D is density, m is mass, and V is volume.
, where D is density, m is mass, and V is volume. - What is the SI unit of pressure according to Gay-Lussac’s law?
A) Newton per square meter (N/m²)
B) Pascal (Pa)
C) Joule (J)
D) Watt (W)
Correct Option: B
Explanation: The SI unit of pressure according to Gay-Lussac’s law is the Pascal (Pa), defined as one Newton per square meter (N/m²). - How does the buoyant force on an object submerged in a fluid change if the density of the object increases?
A) The buoyant force increases.
B) The buoyant force decreases.
C) The buoyant force remains constant.
D) The buoyant force becomes zero.
Correct Option: B
Explanation: An increase in the density of the object results in less displacement of fluid, causing a decrease in the buoyant force. - How does the pressure of a gas change if its temperature remains constant while the volume decreases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Boyle’s law, if the temperature of a gas remains constant, its pressure increases proportionally with a decrease in volume. - What is the formula for pressure according to Gay-Lussac’s law?
A)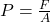
B)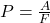
C)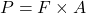
D)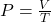
Correct Option: D
Explanation: The formula for pressure according to Gay-Lussac’s law is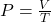 , where P is pressure, V is volume, and T is temperature.
, where P is pressure, V is volume, and T is temperature. - What is the formula for pressure according to Boyle’s law?
A)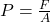
B)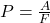
C)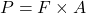
D)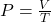
Correct Option: C
Explanation: The formula for pressure according to Boyle’s law is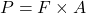 , where P is pressure, F is force, and A is area.
, where P is pressure, F is force, and A is area. - How does the pressure of a gas vary with altitude in the Earth’s atmosphere?
A) Pressure decreases with altitude.
B) Pressure remains constant with altitude.
C) Pressure increases with altitude.
D) Pressure varies unpredictably with altitude.
Correct Option: A
Explanation: Pressure decreases with increasing altitude in the Earth’s atmosphere due to the decreasing density of air molecules. - What is the effect of increasing the volume of a gas on its pressure, according to Boyle’s law?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: A
Explanation: According to Boyle’s law, if the temperature of a gas remains constant, its pressure decreases proportionally with an increase in volume. - Which of the following best describes the relationship between force and deformation in elastic materials?
A) Force is directly proportional to deformation.
B) Force is inversely proportional to deformation.
C) Force is not related to deformation.
D) Force is equal to deformation.
Correct Option: A
Explanation: According to Hooke’s law, force is directly proportional to deformation in elastic materials as long as they remain within their elastic limit. - What is the primary factor determining the pressure exerted by a gas on the walls of its container?
A) Temperature
B) Volume
C) Density
D) Height
Correct Option: B
Explanation: The pressure exerted by a gas on the walls of its container is primarily determined by its volume, as higher volumes of gas exert greater pressure. - How does the pressure of a gas change if its volume remains constant while its temperature increases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Gay-Lussac’s law, if the volume of a gas remains constant, its pressure increases proportionally with an increase in temperature. - What is the formula for density according to Archimedes’ principle?
A)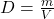
B)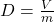
C)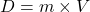
D)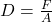
Correct Option: A
Explanation: The formula for density according to Archimedes’ principle is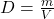 , where D is density, m is mass, and V is volume.
, where D is density, m is mass, and V is volume. - What is the SI unit of pressure according to Gay-Lussac’s law?
A) Newton per square meter (N/m²)
B) Pascal (Pa)
C) Joule (J)
D) Watt (W)
Correct Option: B
Explanation: The SI unit of pressure according to Gay-Lussac’s law is the Pascal (Pa), defined as one Newton per square meter (N/m²). - How does the buoyant force on an object submerged in a fluid change if the density of the object increases?
A) The buoyant force increases.
B) The buoyant force decreases.
C) The buoyant force remains constant.
D) The buoyant force becomes zero.
Correct Option: B
Explanation: An increase in the density of the object results in less displacement of fluid, causing a decrease in the buoyant force. - How does the pressure of a gas change if its temperature remains constant while the volume decreases?
A) Pressure decreases
B) Pressure remains constant
C) Pressure increases
D) Pressure becomes zero
Correct Option: C
Explanation: According to Boyle’s law, if the temperature of a gas remains constant, its pressure increases proportionally with a decrease in volume. - What is the formula for pressure according to Gay-Lussac’s law?
A)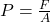
B)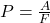
C)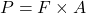
D)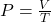
Correct Option: D
Explanation: The formula for pressure according to Gay-Lussac’s law is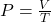 , where P is pressure, V is volume, and T is temperature.
, where P is pressure, V is volume, and T is temperature.
Physics Class 9 MCQs Chapter wise
Explore specialized chapters featuring Class 9 Physics MCQs, meticulously sorted by subject for effortless browsing and focused practice. Each chapter contains over 1000 MCQs, offering ample opportunities for honing skills and mastering Physics concepts effectively. Access MCQs for each chapter easily by selecting the corresponding chapter names listed below.

0 Comments