Explore the our diverse range of 1000+ Thermal Properties of Matter MCQs, accompanied by explanations, tailored for Class 9 students to understand Specific heat Capacity, Change of State, Latent Heat of Fusion etc. principles comprehensively.
100+ Thermal Properties of Matter MCQs with Explanations for Class 9
Discover the intricacies of Thermal Properties of Matter through our specialized collection of MCQs. Tailored for Class 9 students, this extensive repository of over 100+ MCQs, accompanied by detailed explanations ensuring a comprehensive understanding.
- Which of the following best defines temperature?
A) The amount of heat energy in an object
B) The measure of the average kinetic energy of particles in an object
C) The measure of the total internal energy of an object
D) The measure of the amount of matter in an object
Correct Option: B
Explanation: Temperature is a measure of the average kinetic energy of particles in an object. It does not depend on the amount of matter present but on the motion of particles. - What is the SI unit of temperature?
A) Kelvin
B) Celsius
C) Fahrenheit
D) Joule
Correct Option: A
Explanation: The SI unit of temperature is Kelvin, denoted by K. - Which type of thermometer is based on the expansion and contraction of a liquid?
A) Mercury thermometer
B) Alcohol thermometer
C) Digital thermometer
D) Bimetallic thermometer
Correct Option: A
Explanation: Mercury thermometer works based on the expansion and contraction of mercury with temperature changes. - What is the specific heat capacity of a substance?
A) The amount of heat energy required to raise the temperature of 1 kg of the substance by 1 Kelvin
B) The amount of heat energy required to raise the temperature of 1 g of the substance by 1 Celsius
C) The ratio of heat energy to the mass of the substance
D) The ratio of heat energy to the volume of the substance
Correct Option: A
Explanation: Specific heat capacity is defined as the amount of heat energy required to raise the temperature of 1 kg of the substance by 1 Kelvin. - Which substance has the highest specific heat capacity?
A) Water
B) Iron
C) Aluminum
D) Gold
Correct Option: A
Explanation: Water has a higher specific heat capacity compared to other substances, making it useful for regulating temperature in various applications. - What happens to the temperature of a substance during a change of state?
A) It decreases
B) It remains constant
C) It increases
D) It fluctuates randomly
Correct Option: B
Explanation: During a change of state, the temperature remains constant until all the substance has converted to the new state. - What is the latent heat of fusion?
A) The heat energy required to change a substance from liquid to gas
B) The heat energy required to change a substance from gas to liquid
C) The heat energy required to change a substance from solid to liquid
D) The heat energy required to change a substance from liquid to solid Correct Option: D
Explanation: Latent heat of fusion is the heat energy required to change a substance from the solid state to the liquid state at its melting point. - Which of the following statements about evaporation is true?
A) Evaporation occurs only at high temperatures
B) Evaporation is a slow process
C) Evaporation occurs only at the surface of a liquid
D) Evaporation increases with increasing humidity
Correct Option: C
Explanation: Evaporation occurs only at the surface of a liquid where molecules with sufficient kinetic energy escape into the surrounding space. - What is thermal expansion?
A) The increase in mass of an object with increasing temperature
B) The increase in volume of an object with increasing temperature
C) The decrease in density of an object with increasing temperature
D) The decrease in pressure of an object with increasing temperature
Correct Option: B
Explanation: Thermal expansion refers to the increase in volume of an object with increasing temperature due to the increased kinetic energy of its particles. - Which material expands the most when heated?
A) Glass
B) Aluminum
C) Steel
D) Rubber
Correct Option: D
Explanation: Rubber exhibits significant expansion when heated due to its flexible molecular structure. - The process of changing a substance directly from a solid to a gas is called:
A) Evaporation
B) Condensation
C) Sublimation
D) Vaporization
Correct Option: C
Explanation: Sublimation is the process where a substance transitions directly from the solid phase to the gas phase without passing through the liquid phase. - Which of the following is an example of sublimation?
A) Melting of ice
B) Boiling of water
C) Formation of dew
D) Conversion of dry ice to carbon dioxide gas
Correct Option: D
Explanation: Dry ice, which is solid carbon dioxide, sublimes directly into the gas phase at room temperature and pressure. - The temperature at which a solid changes into a liquid is called:
A) Melting point
B) Boiling point
C) Freezing point
D) Condensation point
Correct Option: A
Explanation: Melting point is the temperature at which a solid changes into a liquid. - Which of the following statements about latent heat is correct?
A) Latent heat is released during a change of state
B) Latent heat is the heat required to raise the temperature of a substance
C) Latent heat affects the temperature of a substance directly
D) Latent heat is independent of the mass of the substance
Correct Option: A
Explanation: Latent heat is the heat energy absorbed or released during a change of state without a corresponding change in temperature. - Which substance has the highest latent heat of vaporization?
A) Water
B) Alcohol
C) Mercury
D) Iron
Correct Option: A
Explanation: Water has a high latent heat of vaporization, which is why it requires a significant amount of heat energy to vaporize compared to other substances. - The phenomenon of contraction in a substance due to cooling is known as:
A) Expansion
B) Conduction
C) Convection
D) Contraction
Correct Option: D
Explanation: Contraction refers to the decrease in volume of a substance due to cooling, opposite to expansion. - The coefficient of linear expansion is defined as:
A) The ratio of the change in length to the original length and the change in temperature
B) The ratio of the change in length to the original length
C) The ratio of the change in temperature to the original length
D) The ratio of the original length to the change in temperature
Correct Option: A
Explanation: The coefficient of linear expansion represents the fractional change in length per degree change in temperature. - Which substance expands more for the same increase in temperature: a solid or a gas?
A) Solid
B) Gas
C) Both expand equally
D) It depends on the substance
Correct Option: B
Explanation: Gases generally expand more for the same increase in temperature compared to solids due to their higher molecular mobility. - What does the term ‘specific heat’ refer to?
A) The amount of heat required to raise the temperature of a substance by 1 Kelvin
B) The amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius
C) The temperature at which a substance melts
D) The temperature at which a substance vaporizes
Correct Option: B
Explanation: Specific heat refers to the amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius. - Which of the following is true regarding thermal expansion?
A) All substances expand equally when heated
B) Liquids do not expand when heated
C) Only solids expand when heated
D) Different substances expand at different rates when heated
Correct Option: D
Explanation: Different substances exhibit different rates of expansion when heated due to variations in their molecular structures. - What is the specific heat capacity of water?
A) 1 J/kg°C
B) 4.18 J/g°C
C) 4200 J/kg°C
D) 0.24 J/g°C
Correct Option: C
Explanation: The specific heat capacity of water is approximately 4200 J/kg°C. - Which of the following statements about thermal expansion is correct?
A) All substances expand uniformly when heated
B) Only gases expand when heated
C) Expansion occurs in all directions
D) Expansion is independent of temperature
Correct Option: C
Explanation: Expansion occurs in all directions when a substance is heated, resulting in an increase in volume. - The temperature at which a liquid changes into a gas is called:
A) Melting point
B) Boiling point
C) Freezing point
D) Condensation point
Correct Option: B
Explanation: Boiling point is the temperature at which a liquid changes into a gas. - The process of changing a substance directly from a gas to a solid is called:
A) Melting
B) Freezing
C) Condensation
D) Sublimation
Correct Option: D
Explanation: Sublimation is the process where a substance transitions directly from the gas phase to the solid phase without passing through the liquid phase. - Which of the following materials has the lowest coefficient of linear expansion?
A) Glass
B) Aluminum
C) Steel
D) Rubber
Correct Option: A
Explanation: Glass has a relatively low coefficient of linear expansion compared to metals like aluminum, steel, and rubber. - The coefficient of volume expansion is defined as:
A) The ratio of the change in volume to the original volume and the change in temperature
B) The ratio of the change in volume to the original volume
C) The ratio of the change in temperature to the original volume
D) The ratio of the original volume to the change in temperature
Correct Option: A
Explanation: The coefficient of volume expansion represents the fractional change in volume per degree change in temperature. - Which of the following statements about latent heat of fusion is correct?
A) It is the same for all substances
B) It depends on the mass of the substance
C) It is independent of the substance
D) It is the heat required to change a substance from gas to liquid
Correct Option: C
Explanation: Latent heat of fusion is specific to each substance and represents the amount of heat required to change a unit mass of the substance from the solid to the liquid state. - Which of the following statements about latent heat of vaporization is true?
A) It is the same for all substances
B) It is independent of the substance
C) It is the heat required to change a substance from liquid to gas
D) It depends on the volume of the substance
Correct Option: C
Explanation: Latent heat of vaporization is specific to each substance and represents the amount of heat required to change a unit mass of the substance from the liquid to the gas state. - Which of the following is an example of thermal expansion?
A) A solid turning into a liquid
B) A liquid turning into a gas
C) A metal rod expanding when heated
D) Ice melting into water
Correct Option: C
Explanation: Thermal expansion refers to the increase in size or volume of a substance when heated, such as the expansion of a metal rod when heated. - Which of the following materials has the highest coefficient of volume expansion?
A) Steel
B) Water
C) Glass
D) Rubber
Correct Option: B
Explanation: Water has a relatively high coefficient of volume expansion compared to other common materials. - What is the SI unit of latent heat?
A) Joule
B) Watt
C) Pascal
D) Newton
Correct Option: A
Explanation: The SI unit of latent heat is the joule (J), which represents energy. - Which of the following statements about thermal expansion is correct?
A) Solids expand more than liquids
B) Liquids expand more than gases
C) Gases do not expand when heated
D) All substances expand equally when heated
Correct Option: A
Explanation: Solids generally expand more than liquids, and liquids expand more than gases when heated due to differences in molecular arrangements. - What is the formula to calculate the change in length of a material due to thermal expansion?
A)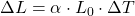
B)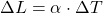
C)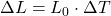
D)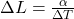
Correct Option: A
Explanation: The formula to calculate the change in length (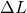 ) of a material due to thermal expansion is given by
) of a material due to thermal expansion is given by 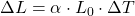 , where
, where 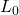 is the original length,
is the original length, 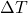 is the change in temperature, and
is the change in temperature, and 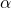 is the coefficient of linear expansion.
is the coefficient of linear expansion. - Which of the following substances has the highest specific heat capacity?
A) Iron
B) Aluminum
C) Water
D) Mercury
Correct Option: C
Explanation: Water has a higher specific heat capacity compared to metals like iron, aluminum, and mercury. - Which of the following is NOT an example of thermal expansion?
A) Expansion joints in bridges
B) Cracking of a metal due to extreme cold
C) Bulging of a tire when heated
D) Melting of ice cream in the sun
Correct Option: D
Explanation: Melting of ice cream in the sun is a phase change phenomenon, not thermal expansion. - Which of the following statements about the specific heat capacity is correct?
A) It depends on the temperature of the substance
B) It is the same for all substances
C) It affects the boiling point of a substance
D) It is measured in grams per degree Celsius
Correct Option: A
Explanation: Specific heat capacity varies with temperature and is specific to each substance. - The coefficient of volume expansion is usually:
A) Greater than the coefficient of linear expansion B) Less than the coefficient of linear expansion
C) Equal to the coefficient of linear expansion
D) Unrelated to the coefficient of linear expansion
Correct Option: A
Explanation: The coefficient of volume expansion is typically greater than the coefficient of linear expansion because volume involves three dimensions, whereas linear expansion involves only one. - Which of the following substances has the highest coefficient of linear expansion?
A) Steel
B) Glass
C) Rubber
D) Water
Correct Option: C
Explanation: Rubber has a relatively high coefficient of linear expansion compared to steel, glass, and water. - What is the formula to calculate the change in volume of a material due to thermal expansion?
A)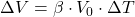
B)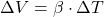
C)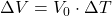
D)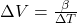
Correct Option: A
Explanation: The formula to calculate the change in volume (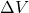 ) of a material due to thermal expansion is given by
) of a material due to thermal expansion is given by 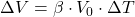 , where
, where 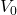 is the original volume,
is the original volume, 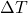 is the change in temperature, and
is the change in temperature, and 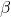 is the coefficient of volume expansion.
is the coefficient of volume expansion. - Which of the following statements about the boiling point of water is true?
A) The boiling point of water is always 100°C
B) The boiling point of water decreases with increasing pressure
C) The boiling point of water increases with increasing altitude
D) The boiling point of water is independent of the amount of water
Correct Option: C
Explanation: The boiling point of water decreases with increasing altitude due to the decrease in atmospheric pressure. - What happens to the density of a substance when it is heated?
A) It decreases
B) It remains constant
C) It increases
D) It depends on the substance
Correct Option: A
Explanation: In general, the density of a substance decreases when it is heated because the volume expands while the mass remains constant. - The phenomenon of thermal expansion is most noticeable in which state of matter?
A) Solid
B) Liquid
C) Gas
D) Plasma
Correct Option: A
Explanation: Thermal expansion is most noticeable in solids due to their rigid structure and closely packed arrangement of particles. - Which of the following materials is commonly used in bimetallic strips?
A) Aluminum and steel
B) Copper and brass
C) Iron and nickel
D) Lead and zinc
Correct Option: C
Explanation: Bimetallic strips are typically made of two different metals with different coefficients of thermal expansion, such as iron and nickel. - Which of the following statements about thermal expansion is correct?
A) All substances expand uniformly when heated
B) Liquids do not expand when heated
C) Only solids expand when heated
D) Expansion occurs in all directions
Correct Option: D
Explanation: Expansion occurs in all directions when a substance is heated, resulting in an increase in volume. - Which of the following materials has the lowest coefficient of volume expansion?
A) Steel
B) Water
C) Glass
D) Rubber
Correct Option: C
Explanation: Glass has a relatively low coefficient of volume expansion compared to steel, water, and rubber. - What is the formula to calculate the change in volume of a material due to thermal expansion?
A)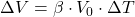
B)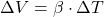
C)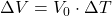
D)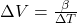
Correct Option: A
Explanation: The formula to calculate the change in volume (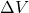 ) of a material due to thermal expansion is given by
) of a material due to thermal expansion is given by 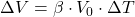 , where
, where 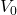 is the original volume,
is the original volume, 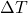 is the change in temperature, and
is the change in temperature, and 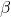 is the coefficient of volume expansion.
is the coefficient of volume expansion. - Which of the following statements about thermal expansion is correct?
A) Solids expand more than liquids
B) Liquids expand more than gases
C) Gases do not expand when heated
D) All substances expand equally when heated
Correct Option: A
Explanation: Solids generally expand more than liquids, and liquids expand more than gases when heated due to differences in molecular arrangements. - What is the SI unit of latent heat?
A) Joule
B) Watt
C) Pascal
D) Newton
Correct Option: A
Explanation: The SI unit of latent heat is the joule (J), which represents energy. - Which of the following statements about thermal expansion is correct?
A) All substances expand uniformly when heated
B) Only gases expand when heated
C) Expansion occurs in all directions
D) Expansion is independent of temperature
Correct Option: C
Explanation: Expansion occurs in all directions when a substance is heated, resulting in an increase in volume. - The temperature at which a liquid changes into a gas is called:
A) Melting point
B) Boiling point
C) Freezing point
D) Condensation point
Correct Option: B
Explanation: Boiling point is the temperature at which a liquid changes into a gas. - The process of changing a substance directly from a gas to a solid is called:
A) Melting
B) Freezing
C) Condensation
D) Sublimation
Correct Option: D
Explanation: Sublimation is the process where a substance transitions directly from the gas phase to the solid phase without passing through the liquid phase. - Which of the following materials has the lowest coefficient of linear expansion?
A) Glass
B) Aluminum
C) Steel
D) Rubber
Correct Option: A
Explanation: Glass has a relatively low coefficient of linear expansion compared to metals like aluminum, steel, and rubber. - The coefficient of volume expansion is defined as:
A) The ratio of the change in volume to the original volume and the change in temperature
B) The ratio of the change in volume to the original volume
C) The ratio of the change in temperature to the original volume
D) The ratio of the original volume to the change in temperature
Correct Option: A
Explanation: The coefficient of volume expansion represents the fractional change in volume per degree change in temperature. - Which substance expands more for the same increase in temperature: a solid or a gas?
A) Solid
B) Gas
C) Both expand equally
D) It depends on the substance
Correct Option: B
Explanation: Gases generally expand more for the same increase in temperature compared to solids due to their higher molecular mobility. - What does the term ‘specific heat’ refer to?
A) The amount of heat required to raise the temperature of a substance by 1 Kelvin
B) The amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius
C) The temperature at which a substance melts
D) The temperature at which a substance vaporizes
Correct Option: B
Explanation: Specific heat refers to the amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius. - Which of the following is true regarding thermal expansion?
A) All substances expand equally when heated
B) Liquids do not expand when heated
C) Only solids expand when heated
D) Different substances expand at different rates when heated
Correct Option: D
Explanation: Different substances exhibit different rates of expansion when heated due to variations in their molecular structures. - What is the specific heat capacity of water?
A) 1 J/kg°C
B) 4.18 J/g°C
C) 4200 J/kg°C
D) 0.24 J/g°C
Correct Option: C
Explanation: The specific heat capacity of water is approximately 4200 J/kg°C. - Which of the following statements about thermal expansion is correct?
A) Solids expand more than liquids
B) Liquids expand more than gases
C) Gases do not expand when heated
D) All substances expand equally when heated
Correct Option: A
Explanation: Solids generally expand more than liquids, and liquids expand more than gases when heated due to differences in molecular arrangements. - The temperature at which a liquid changes into a gas is called:
A) Melting point
B) Boiling point
C) Freezing point
D) Condensation point
Correct Option: B
Explanation: Boiling point is the temperature at which a liquid changes into a gas. - The process of changing a substance directly from a gas to a solid is called:
A) Melting
B) Freezing
C) Condensation
D) Sublimation
Correct Option: D
Explanation: Sublimation is the process where a substance transitions directly from the gas phase to the solid phase without passing through the liquid phase. - Which of the following materials has the lowest coefficient of linear expansion?
A) Glass
B) Aluminum
C) Steel
D) Rubber
Correct Option: A
Explanation: Glass has a relatively low coefficient of linear expansion compared to metals like aluminum, steel, and rubber. - The coefficient of volume expansion is defined as:
A) The ratio of the change in volume to the original volume and the change in temperature
B) The ratio of the change in volume to the original volume
C) The ratio of the change in temperature to the original volume
D) The ratio of the original volume to the change in temperature
Correct Option: A
Explanation: The coefficient of volume expansion represents the fractional change in volume per degree change in temperature. - Which substance expands more for the same increase in temperature: a solid or a gas?
A) Solid
B) Gas
C) Both expand equally
D) It depends on the substance
Correct Option: B
Explanation: Gases generally expand more for the same increase in temperature compared to solids due to their higher molecular mobility. - What does the term ‘specific heat’ refer to?
A) The amount of heat required to raise the temperature of a substance by 1 Kelvin
B) The amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius
C) The temperature at which a substance melts
D) The temperature at which a substance vaporizes
Correct Option: B
Explanation: Specific heat refers to the amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius. - Which of the following is true regarding thermal expansion?
A) All substances expand equally when heated
B) Liquids do not expand when heated
C) Only solids expand when heated
D) Different substances expand at different rates when heated
Correct Option: D
Explanation: Different substances exhibit different rates of expansion when heated due to variations in their molecular structures. - What is the specific heat capacity of water?
A) 1 J/kg°C
B) 4.18 J/g°C
C) 4200 J/kg°C
D) 0.24 J/g°C
Correct Option: C
Explanation: The specific heat capacity of water is approximately 4200 J/kg°C. - Which of the following statements about thermal expansion is correct?
A) Solids expand more than liquids
B) Liquids expand more than gases
C) Gases do not expand when heated
D) All substances expand equally when heated
Correct Option: A
Explanation: Solids generally expand more than liquids, and liquids expand more than gases when heated due to differences in molecular arrangements. - The temperature at which a liquid changes into a gas is called:
A) Melting point
B) Boiling point
C) Freezing point
D) Condensation point
Correct Option: B
Explanation: Boiling point is the temperature at which a liquid changes into a gas. - The process of changing a substance directly from a gas to a solid is called:
A) Melting
B) Freezing
C) Condensation
D) Sublimation
Correct Option: D
Explanation: Sublimation is the process where a substance transitions directly from the gas phase to the solid phase without passing through the liquid phase. - Which of the following materials has the lowest coefficient of linear expansion?
A) Glass
B) Aluminum
C) Steel
D) Rubber
Correct Option: A
Explanation: Glass has a relatively low coefficient of linear expansion compared to metals like aluminum, steel, and rubber. - The coefficient of volume expansion is defined as:
A) The ratio of the change in volume to the original volume and the change in temperature
B) The ratio of the change in volume to the original volume
C) The ratio of the change in temperature to the original volume
D) The ratio of the original volume to the change in temperature
Correct Option: A
Explanation: The coefficient of volume expansion represents the fractional change in volume per degree change in temperature. - Which substance expands more for the same increase in temperature: a solid or a gas?
A) Solid
B) Gas
C) Both expand equally
D) It depends on the substance
Correct Option: B
Explanation: Gases generally expand more for the same increase in temperature compared to solids due to their higher molecular mobility. - What does the term ‘specific heat’ refer to?
A) The amount of heat required to raise the temperature of a substance by 1 Kelvin
B) The amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius
C) The temperature at which a substance melts
D) The temperature at which a substance vaporizes
Correct Option: B
Explanation: Specific heat refers to the amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius. - Which of the following is true regarding thermal expansion?
A) All substances expand equally when heated
B) Liquids do not expand when heated
C) Only solids expand when heated
D) Different substances expand at different rates when heated
Correct Option: D
Explanation: Different substances exhibit different rates of expansion when heated due to variations in their molecular structures. - What is the specific heat capacity of water?
A) 1 J/kg°C
B) 4.18 J/g°C
C) 4200 J/kg°C
D) 0.24 J/g°C
Correct Option: C
Explanation: The specific heat capacity of water is approximately 4200 J/kg°C. - Which of the following statements about thermal expansion is correct?
A) Solids expand more than liquids
B) Liquids expand more than gases
C) Gases do not expand when heated
D) All substances expand equally when heated
Correct Option: A
Explanation: Solids generally expand more than liquids, and liquids expand more than gases when heated due to differences in molecular arrangements. - The temperature at which a liquid changes into a gas is called:
A) Melting point
B) Boiling point
C) Freezing point
D) Condensation point
Correct Option: B
Explanation: Boiling point is the temperature at which a liquid changes into a gas. - The process of changing a substance directly from a gas to a solid is called:
A) Melting
B) Freezing
C) Condensation
D) Sublimation
Correct Option: D
Explanation: Sublimation is the process where a substance transitions directly from the gas phase to the solid phase without passing through the liquid phase. - Which of the following materials has the lowest coefficient of linear expansion?
A) Glass
B) Aluminum
C) Steel
D) Rubber
Correct Option: A
Explanation: Glass has a relatively low coefficient of linear expansion compared to metals like aluminum, steel, and rubber. - The coefficient of volume expansion is defined as:
A) The ratio of the change in volume to the original volume and the change in temperature
B) The ratio of the change in volume to the original volume
C) The ratio of the change in temperature to the original volume
D) The ratio of the original volume to the change in temperature
Correct Option: A
Explanation: The coefficient of volume expansion represents the fractional change in volume per degree change in temperature. - Which substance expands more for the same increase in temperature: a solid or a gas?
A) Solid
B) Gas
C) Both expand equally
D) It depends on the substance
Correct Option: B
Explanation: Gases generally expand more for the same increase in temperature compared to solids due to their higher molecular mobility. - What does the term ‘specific heat’ refer to?
A) The amount of heat required to raise the temperature of a substance by 1 Kelvin
B) The amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius
C) The temperature at which a substance melts
D) The temperature at which a substance vaporizes
Correct Option: B
Explanation: Specific heat refers to the amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius. - Which of the following is true regarding thermal expansion?
A) All substances expand equally when heated
B) Liquids do not expand when heated
C) Only solids expand when heated
D) Different substances expand at different rates when heated
Correct Option: D
Explanation: Different substances exhibit different rates of expansion when heated due to variations in their molecular structures. - What is the specific heat capacity of water?
A) 1 J/kg°C
B) 4.18 J/g°C
C) 4200 J/kg°C
D) 0.24 J/g°C
Correct Option: C
Explanation: The specific heat capacity of water is approximately 4200 J/kg°C. - Which of the following statements about thermal expansion is correct?
A) Solids expand more than liquids
B) Liquids expand more than gases
C) Gases do not expand when heated
D) All substances expand equally when heated
Correct Option: A
Explanation: Solids generally expand more than liquids, and liquids expand more than gases when heated due to differences in molecular arrangements. - The temperature at which a liquid changes into a gas is called:
A) Melting point
B) Boiling point
C) Freezing point
D) Condensation point
Correct Option: B
Explanation: Boiling point is the temperature at which a liquid changes into a gas. - The process of changing a substance directly from a gas to a solid is called:
A) Melting
B) Freezing
C) Condensation
D) Sublimation
Correct Option: D
Explanation: Sublimation is the process where a substance transitions directly from the gas phase to the solid phase without passing through the liquid phase. - Which of the following materials has the lowest coefficient of linear expansion?
A) Glass
B) Aluminum
C) Steel
D) Rubber
Correct Option: A
Explanation: Glass has a relatively low coefficient of linear expansion compared to metals like aluminum, steel, and rubber. - The coefficient of volume expansion is defined as:
A) The ratio of the change in volume to the original volume and the change in temperature
B) The ratio of the change in volume to the original volume
C) The ratio of the change in temperature to the original volume
D) The ratio of the original volume to the change in temperature
Correct Option: A
Explanation: The coefficient of volume expansion represents the fractional change in volume per degree change in temperature. - Which substance expands more for the same increase in temperature: a solid or a gas?
A) Solid
B) Gas
C) Both expand equally
D) It depends on the substance
Correct Option: B
Explanation: Gases generally expand more for the same increase in temperature compared to solids due to their higher molecular mobility. - What does the term ‘specific heat’ refer to?
A) The amount of heat required to raise the temperature of a substance by 1 Kelvin
B) The amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius
C) The temperature at which a substance melts
D) The temperature at which a substance vaporizes
Correct Option: B
Explanation: Specific heat refers to the amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius. - Which of the following is true regarding thermal expansion?
A) All substances expand equally when heated
B) Liquids do not expand when heated
C) Only solids expand when heated
D) Different substances expand at different rates when heated
Correct Option: D
Explanation: Different substances exhibit different rates of expansion when heated due to variations in their molecular structures. - What is the specific heat capacity of water?
A) 1 J/kg°C
B) 4.18 J/g°C
C) 4200 J/kg°C
D) 0.24 J/g°C
Correct Option: C
Explanation: The specific heat capacity of water is approximately 4200 J/kg°C. - Which of the following statements about thermal expansion is correct?
A) Solids expand more than liquids
B) Liquids expand more than gases
C) Gases do not expand when heated
D) All substances expand equally when heated
Correct Option: A
Explanation: Solids generally expand more than liquids, and liquids expand more than gases when heated due to differences in molecular arrangements. - The temperature at which a liquid changes into a gas is called:
A) Melting point
B) Boiling point
C) Freezing point
D) Condensation point
Correct Option: B
Explanation: Boiling point is the temperature at which a liquid changes into a gas. - The process of changing a substance directly from a gas to a solid is called:
A) Melting
B) Freezing
C) Condensation
D) Sublimation
Correct Option: D
Explanation: Sublimation is the process where a substance transitions directly from the gas phase to the solid phase without passing through the liquid phase. - Which of the following materials has the lowest coefficient of linear expansion?
A) Glass
B) Aluminum
C) Steel
D) Rubber
Correct Option: A
Explanation: Glass has a relatively low coefficient of linear expansion compared to metals like aluminum, steel, and rubber. - The coefficient of volume expansion is defined as:
A) The ratio of the change in volume to the original volume and the change in temperature
B) The ratio of the change in volume to the original volume
C) The ratio of the change in temperature to the original volume
D) The ratio of the original volume to the change in temperature
Correct Option: A
Explanation: The coefficient of volume expansion represents the fractional change in volume per degree change in temperature. - Which substance expands more for the same increase in temperature: a solid or a gas?
A) Solid
B) Gas
C) Both expand equally
D) It depends on the substance
Correct Option: B
Explanation: Gases generally expand more for the same increase in temperature compared to solids due to their higher molecular mobility. - What does the term ‘specific heat’ refer to?
A) The amount of heat required to raise the temperature of a substance by 1 Kelvin
B) The amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius
C) The temperature at which a substance melts
D) The temperature at which a substance vaporizes
Correct Option: B
Explanation: Specific heat refers to the amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius. - Which of the following is true regarding thermal expansion?
A) All substances expand equally when heated
B) Liquids do not expand when heated
C) Only solids expand when heated
D) Different substances expand at different rates when heated
Correct Option: D
Explanation: Different substances exhibit different rates of expansion when heated due to variations in their molecular structures. - What is the specific heat capacity of water?
A) 1 J/kg°C
B) 4.18 J/g°C
C) 4200 J/kg°C
D) 0.24 J/g°C
Correct Option: C
Explanation: The specific heat capacity of water is approximately 4200 J/kg°C. - Which of the following statements about thermal expansion is correct?
A) Solids expand more than liquids
B) Liquids expand more than gases
C) Gases do not expand when heated
D) All substances expand equally when heated
Correct Option: A
Explanation: Solids generally expand more than liquids, and liquids expand more than gases when heated due to differences in molecular arrangements. - The temperature at which a liquid changes into a gas is called:
A) Melting point
B) Boiling point
C) Freezing point
D) Condensation point
Correct Option: B
Explanation: Boiling point is the temperature at which a liquid changes into a gas. - The process of changing a substance directly from a gas to a solid is called:
A) Melting
B) Freezing
C) Condensation
D) Sublimation
Correct Option: D
Explanation: Sublimation is the process where a substance transitions directly from the gas phase to the solid phase without passing through the liquid phase. - Which of the following materials has the lowest coefficient of linear expansion?
A) Glass
B) Aluminum
C) Steel
D) Rubber
Correct Option: A
Explanation: Glass has a relatively low coefficient of linear expansion compared to metals like aluminum, steel, and rubber. - The coefficient of volume expansion is defined as:
A) The ratio of the change in volume to the original volume and the change in temperature
B) The ratio of the change in volume to the original volume
C) The ratio of the change in temperature to the original volume
D) The ratio of the original volume to the change in temperature
Correct Option: A
Explanation: The coefficient of volume expansion represents the fractional change in volume per degree change in temperature. - Which substance expands more for the same increase in temperature: a solid or a gas?
A) Solid
B) Gas
C) Both expand equally
D) It depends on the substance
Correct Option: B
Explanation: Gases generally expand more for the same increase in temperature compared to solids due to their higher molecular mobility. - What does the term ‘specific heat’ refer to?
A) The amount of heat required to raise the temperature of a substance by 1 Kelvin
B) The amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius
C) The temperature at which a substance melts
D) The temperature at which a substance vaporizes
Correct Option: B
Explanation: Specific heat refers to the amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius.
110. Which of the following is true regarding thermal expansion?
A) All substances expand equally when heated
B) Liquids do not expand when heated
C) Only solids expand when heated
D) Different substances expand at different rates when heated
Correct Option: D
Explanation: Different substances exhibit different rates of expansion when heated due to variations in their molecular structures.
Physics Class 9 MCQs Chapter wise
Delve into exclusive sections showcasing Class 9 Physics MCQs, systematically categorized by topic for convenient exploration and concentrated study. Each section features over 1000 MCQs, presenting ample practice opportunities to enhance understanding and excel in Physics. Access MCQs corresponding to each section by simply tapping on the provided chapter titles.

0 Comments